Your Location:Home > Products > chemicals for Water treatment > Trichloroisocyanuric acid(TCCA)
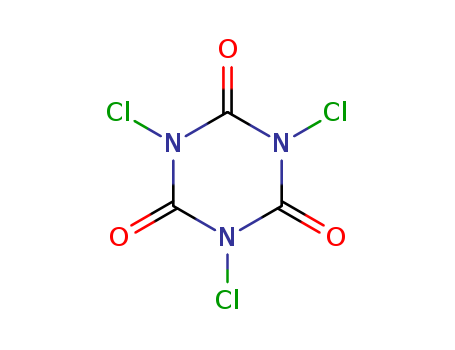


CasNo: 87-90-1
MF: C3Cl3N3O3
Appearance: white to light yellow crystal powder
|
Chemical Description |
Trichloroisocyanuric acid is a chlorinated derivative of isocyanuric acid. |
|
Bactericidal and Disinfection effect |
Trichloroisocyanuric acid (TCCA) is a strong oxidant and chlorinating agent which has efficient, broad-spectrum, and safe disinfection effects. It has the strongest bactericidal activity among chlorinated isocyanuric acid products against bacteria, viruses, fungi, mold, Vibrio cholerae, Bacillus. It also has certain inhibitory effect on coccidia oocysts and thus can used in the disinfection in the environment, drinking water, fruits and vegetables, livestock feeders, fish ponds and silkworm rooms. The application method is using powder for the preparation of 4-6 mg/kg concentrations for the disinfection of drinking water and using a solution of concentration of 200-400 mg/kg for environmental disinfection and utensils disinfection. TCCA has various effects including killing algae, deodorant, and water purification as well as bleaching. It has a stronger and better bactericidal and bleaching effect than sodium dichloroisocyanurate and thus being widely used in the sterilization treatment of washing bleacher of cotton and hemp fiber fabric, wool shrink proofing agents, rubber chlorination, and oil drilling mud and sewage, disinfection of battery materials, disinfection of swimming pool, disinfection of drinking water, treatment of industrial wastewater and sewage, food processing industry, food hygiene industry, aquaculture, daily chemical industry, hospitals, nurseries, epidemic prevention, waste disposal, hotels, restaurants and natural disasters, large-scale disinfection and sterilization after man-made disaster. Instructions: 1. swimming pool disinfection: calculate the usage according to 2-3g/m3; take effect for 30-60 minutes. 2. Public places and ground disinfection: use 1500-3000-fold diluted water solution for spraying and wiping for 30 minutes. Infectious disease epidemic area, use 150-200-fold aqueous diluted water solution for spray disinfection. 3. disinfection of white clothing and blankets: apply 2000-3000 fold diluted solution for clean items; soak for 10 minutes; for seriously polluted items, diluted 500 times, soak for 20 minutes. 4. disinfection of bathroom and toilet scrub; use 300-fold diluted solution for reaction of 30 minutes. 5. sterilization of non-metallic medical equipment: use 500-1000 times diluted solution for soak of 30 minutes; disinfection of apparatus with pus and blood; apply 100-fold diluted water solution for reaction of 30 minutes. 6. disinfection of non-metallic tableware: use 500-1000 times diluted water solution for reaction of 30 minutes. |
|
Acute toxicity |
oral: rat LD50: 406 mg/kg |
|
Irritation data |
skin: rabbit 500 mg/24 hours: moderate; Eyes-rabbit 50 micrograms/24 hours and severe. |
|
Explosiveness and Hazardous characteristics |
being mixed with ammonium salt, ammonia, urea can generate explosive nitrogen trichloride. |
|
Flammability and hazard characteristics |
it is flammable in case of organic substance; it can generate toxic nitrogen trichloride upon wet and heating. |
|
Storage characteristics |
Treasury: ventilation, low-temperature and dry; it should be stored separately from fuel, organic matter, and oxides. |
|
Fire extinguishing agent |
Plenty of water |
|
General Description |
A white slightly hygroscopic crystalline powder or lump solid with a mild chlorine-like odor. Said to have 85 percent available chlorine. Decomposes at 225°C. Moderately toxic by ingestion. May irritate skin and eyes. Active ingredient in household dry bleaches. Used in swimming pools as a disinfectant . |
|
Air & Water Reactions |
Slightly hygroscopic [Hawley]. Slightly soluble in water. May react with water releasing gaseous chlorine. If mixed with a small amount of water, the concentrated solution (with pH at about 2.0) may explode due to the evolution of unstable nitrogen trichloride. (explanation in Bretherick 5th ed.) |
|
Reactivity Profile |
TRICHLORO-S-TRIAZINETRIONE, [DRY, CONTAINING > 39% AVAILABLE CHLORINE] may react vigorously with ammonium compounds or hydrated salts. Prolonged exposure to heat may result in the vigorous decomposition with the rupture of containers. Accelerates the burning of combustible materials [AAR 1991]. May reactwith alcohols to yield alkyl hypochlorites that decompose in the cold and explode on exposure to sunlight or heat. Hypochlorites of tertiary alcohols are less unstable than those of secondary or primary alcohols [NFPA 491 M. 1991]. |
|
Health Hazard |
Inhalation causes sneezing and coughing. Contact with dust causes moderate irritation of eyes and itching and redness of skin. Ingestion causes burns of mouth and stomach. |
|
Safety Profile |
Moderately toxic to humans and experimentally by ingestion. Mildly toxic experimentally by skin contact. Human systemic effects by ingestion: ulceration or bleeding from stomach. A severe skin and eye irritant. Toxicity symptoms include emaciation, lethargy, weakness, and delayed death. Autopsy shows inflammation of gastrointestinal tract, liver discoloration, and kidney hyperemia. A powerful oxidizer. Forms an explosive product with cyanuric acid + sodmm hydroxide. Potentially violent reaction with combustible materials. When heated to decomposition it emits very toxic fumes of Cland NOx. Used to chlorinate swimming pools. |
InChI:InChI=1/C3Cl3N3O3/c4-7-1(10)8(5)3(12)9(6)2(7)11
The invention relates to a preparation m...
The invention discloses a preparation me...
The invention relates to the field of ch...
The invention belongs to the technical f...
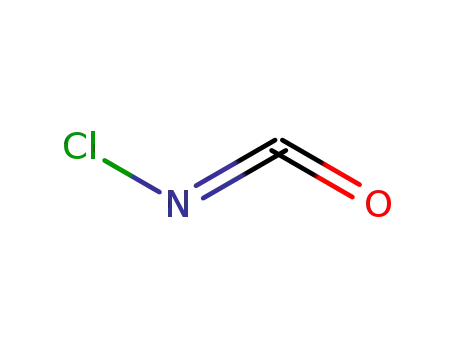
chlorine isocyanate

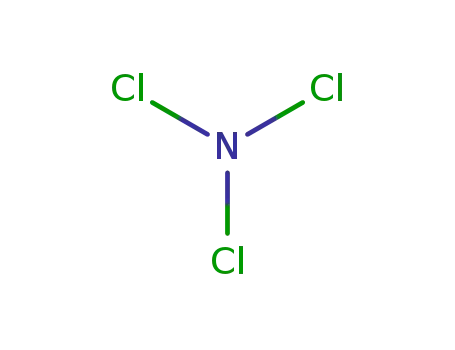
nitrogen trichloride

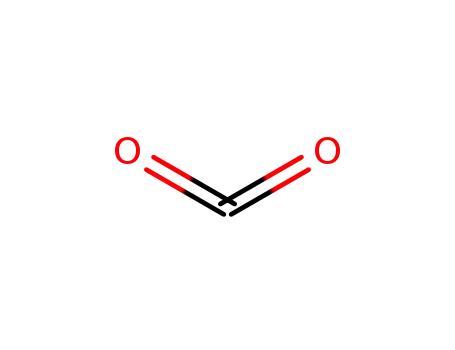
carbon dioxide

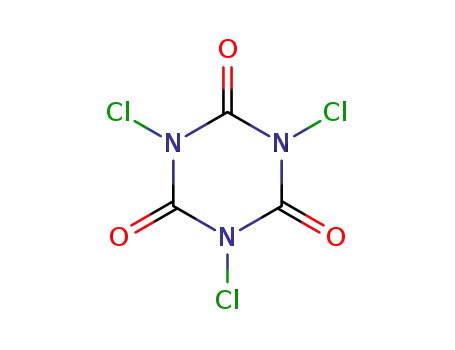
trichloroisocyanuric acid

![1,3-dichloro-[1,3,5]triazinane-2,4,6-trione](/upload/2025/4/78110c1d-4323-4524-9051-41f648195083.png)
1,3-dichloro-[1,3,5]triazinane-2,4,6-trione

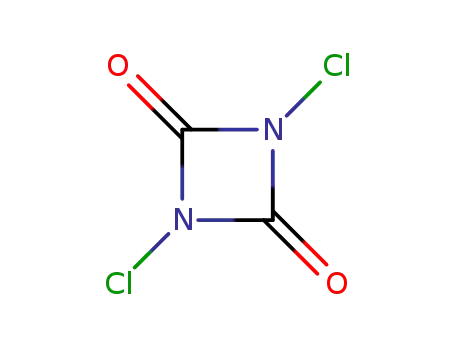
1,3-dichloro-1,3-diazetidine-2,4-dione
| Conditions | Yield |
|---|---|
|
slow react. above the melting point, fast at room temp., dichlorouretidindione formed by vac. sublimation (50.degrre.C, 0.1 Torr), di- and trichlocyanuric acid yielded by 48h sublimation of the residue at 95-160°C;
|
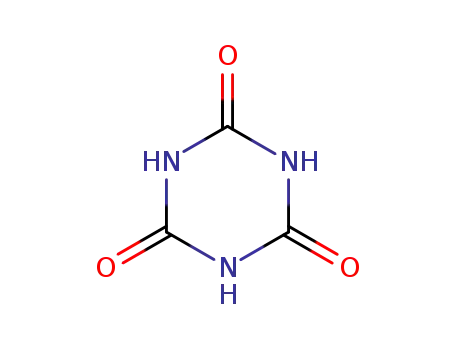
cyanuric acid


trichloroisocyanuric acid
| Conditions | Yield |
|---|---|
|
With
chlorine; calcium carbonate;
In
water;
|
100% |
|
With
hypochlorous anhydride;
In
water;
at 25 ℃;
for 6h;
pH=3.5;
pH-value;
Temperature;
Large scale;
|
98% |
|
With
sodium hydroxide; chlorine;
unter der Einwirkung von UV-Licht;
|
|
|
With
potassium hydroxide; chlorine;
unter der Einwirkung von UV-LIcht;
|

cyanuric acid
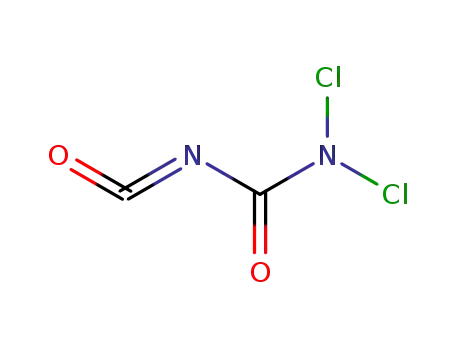
N'-carbonyl-N,N-dichloro-urea
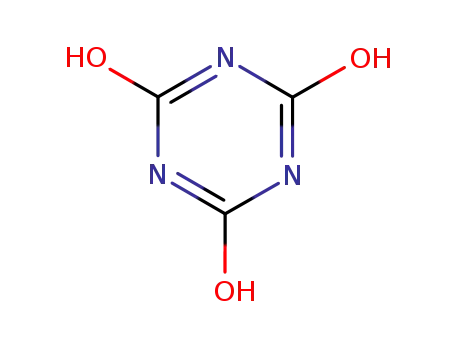
isocyanuric acid
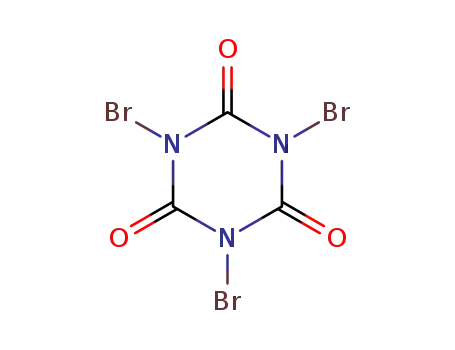
1,3,5-tribromo-1,3,5-triazinane-2,4,6-trione
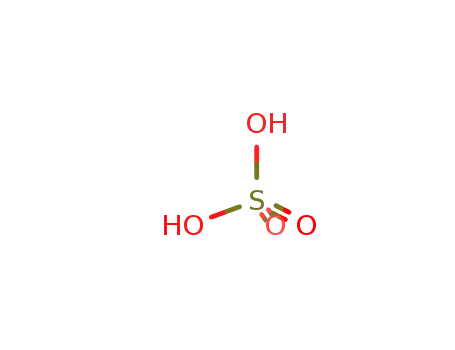
sulfuric acid

isocyanuric acid
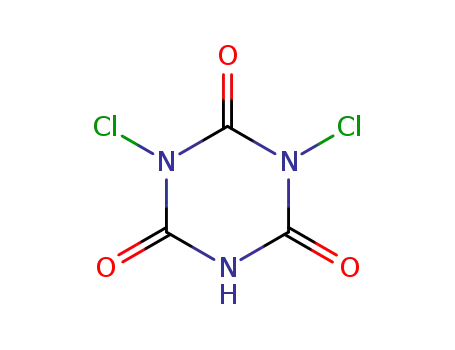
1,3-dichloro-[1,3,5]triazinane-2,4,6-trione
4,4'-dimethoxyphenyl disulfide