Your Location:Home > Products > chemicals for Plastic additives > Dioctyl phthalate(DOP)
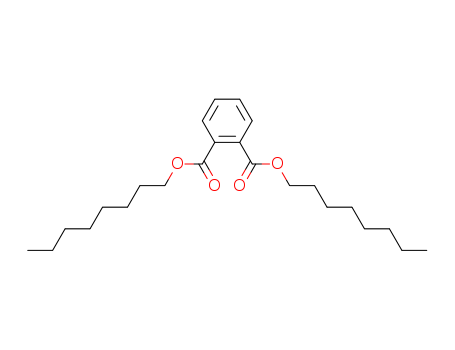


CasNo: 117-84-0
MF: C24H38O4
Appearance: clear liquid with a mild odor
|
Physical properties |
Clear, light colored, viscous, oily liquid with a slight odor |
|
General Description |
A clear liquid with a mild odor. Slightly less dense than water and insoluble in water. Hence floats on water. Flash point 430°F. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. As a liquid, can easily penetrate the soil and contaminate groundwater and nearby streams. Eye contact may produce severe irritation and direct skin contact may produce mild irritation. Used in the manufacture of a variety of plastics and coating products. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
DI-N-OCTYL PHTHALATE reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen may be generated by mixing with alkali metals and hydrides. Can generate electrostatic charges by swirling or pouring [Handling Chemicals Safely, 1980. p. 250]. |
|
Health Hazard |
Produces no ill effects at normal temperatures but may give off irritating vapor at high temperature. |
|
Fire Hazard |
Special Hazards of Combustion Products: None |
|
Safety Profile |
Mildly toxic by ingestion. Experimental teratogenic and reproductive effects. A skin and severe eye irritant. Used as a plasticizer. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS. |
|
Source |
Detected in distilled water-soluble fractions of new and used motor oil at concentrations of 1.3 to 1.4 and 73 to 78 μg/L, respectively (Chen et al., 1994). May leach from plastic products (e.g., tubing, containers) used in laboratories during chemical analysis of aqueous samples. |
|
Environmental fate |
Biological. o-Phthalic acid was tentatively identified as the major degradation product of di-noctyl phthalate produced by the bacterium Serratia marcescens (Mathur and Rouatt, 1975). When di-n-octyl phthalate was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, no degradation was observed after 7 d. In a 21-d period, however, gradual adaptation did occur, resulting in 94 and 93% losses at concentrations of 5 and 10 mg/L, respectively (Tabak et al., 1981). In the presence of suspended natural populations from unpolluted aquatic systems, the second-order microbial transformation rate constant determined in the laboratory was reported to be 3.7 ± 0.6 x 10-13 L/organism?h (Steen, 1991). Chemical/Physical. Under alkaline conditions, di-n-octyl phthalate will initially hydrolyze to noctyl hydrogen phthalate and 1-octanol. The monoester will undergo hydrolysis forming ophthalic acid and 1-octanol (Kollig, 1993). The hydrolysis half-life at pH 7 and 25 °C was estimated to be 107 yr (Ellington et al., 1988). |
InChI:InChI=1/C24H38O4/c1-3-5-7-9-11-15-19-27-23(25)21-17-13-14-18-22(21)24(26)28-20-16-12-10-8-6-4-2/h13-14,17-18H,3-12,15-16,19-20H2,1-2H3
Abstract: A mechanism for the degradatio...
The invention relates to a production pr...
The present study reports the synthesis ...
The present invention relates to a novel...
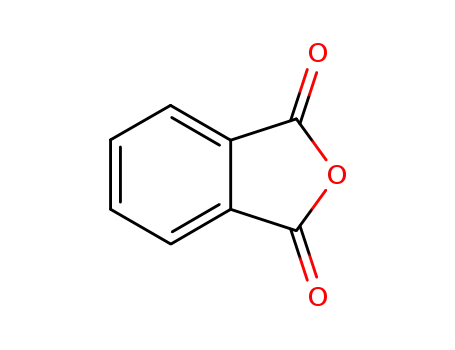
phthalic anhydride

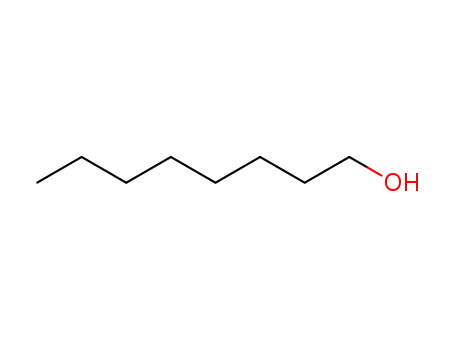
octanol

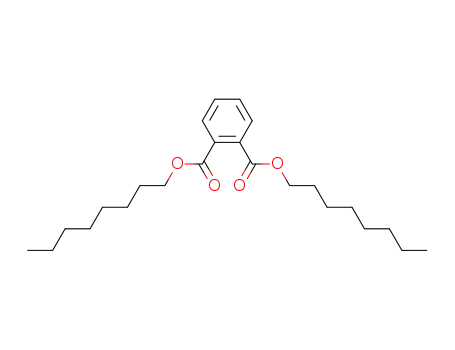
Di-n-octyl phthalate

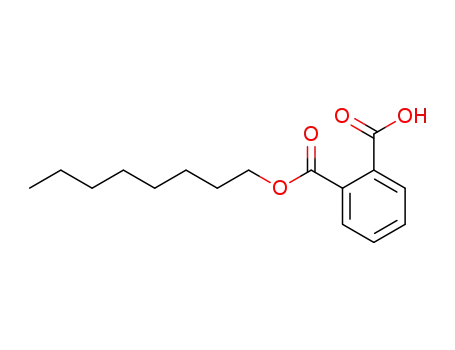
Mono-n-octyl phthalate
| Conditions | Yield |
|---|---|
|
With
montmorillonite K-10;
In
tetrachloromethane;
at 76 ℃;
for 12h;
|
54% 21% |
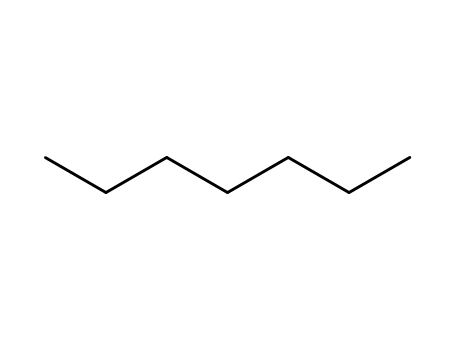
n-heptane

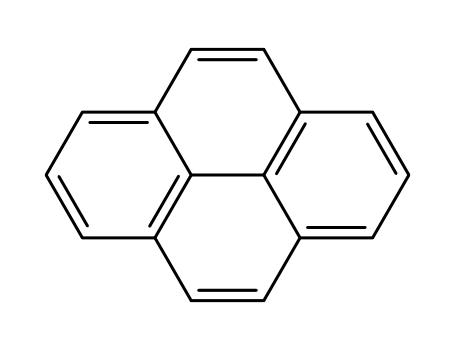
pyrene

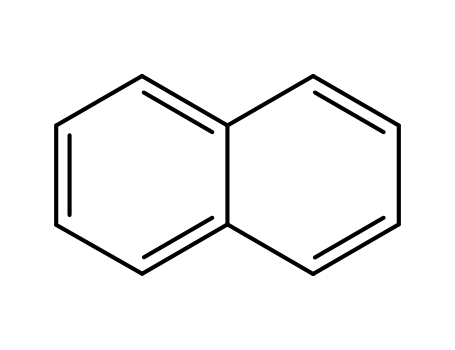
naphthalene

biphenyl

2-phenylnaphthalene

phenanthrene

anthracene


fluoranthene


Di-n-octyl phthalate

acenaphthene

acenaphthylene
| Conditions | Yield |
|---|---|
|
Mechanism;
Microwave irradiation;
|

phthalic anhydride

octanol

Diethyl phthalate
benzene-1,2-dicarboxylic acid

benzene-1,2-dicarboxylic acid

Mono-n-octyl phthalate
4-methyl-tetrahydro-pyran-2-one
3-methylpentane-1,5-diol