Your Location:Home > Products > chemicals for Detergent > Boric acid
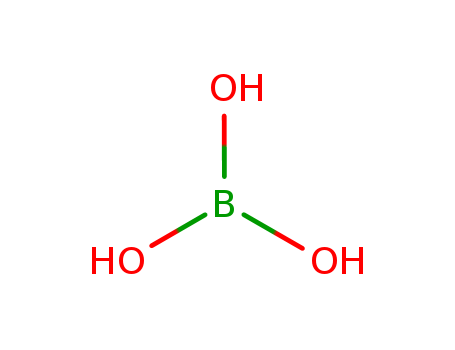


CasNo: 10043-35-3
MF: BH3O3
Appearance: white crystalline solid
|
General Description |
Boric acid, also known as hydrogen borate, is a weak and monobasic acid of boron, often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other chemical compounds. With the chemical formula B(OH)3, it contains the elements boron, hydrogen, and oxygen. It naturally occurs in some volcanic spring waters or can be manufactured from borax. It is solid under room temperature, appearing as colorless crystals or a white powder, and can be dissolved in water. Breathing in boric acid can irritate the respiratory system and higher or chronic exposure could bring bigger health risks. |
InChI:InChI=1/BH3O3/c2-1(3)4/h2-4H
The crystal structure of H3BO3-3T, a new...
Single crystals of Li3[B8O12(OH) 3] were...
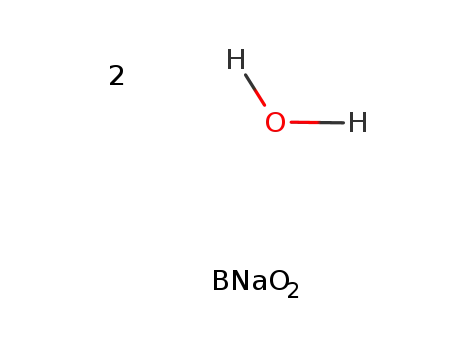
sodium metaborate dihydrate

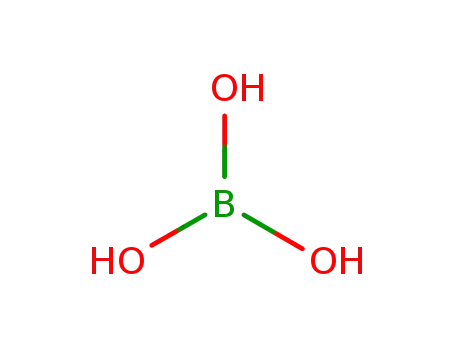
orthoboric acid

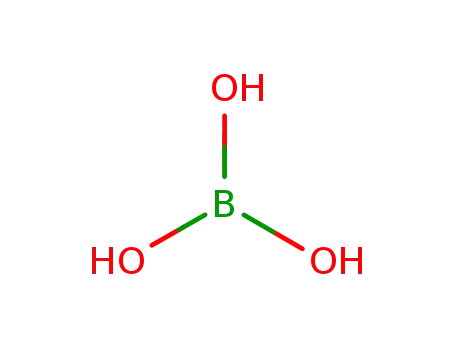
orthoboric acid
| Conditions | Yield |
|---|---|
|
With
UO3;
In
water;
High Pressure; mild hydrothermal synthesis; NaBO2(H2O)2, UO3 (4:1 mol) dissolved in ultrapure H2O; pH 0.5 adjusted with concd. HNO3 (15.4 M); soln. placed in Teflon-lined Parr bomb; heated at 453 K, 48 h; cooled to ambient temp.; evapd. in a fume hood; after 2 d crystals of H3BO3-2A (triclinic) and H3BO3-3T (tetragonal) were recovered;
|

sodium metaborate dihydrate


orthoboric acid


orthoboric acid
| Conditions | Yield |
|---|---|
|
With
UO3;
In
water;
High Pressure; mild hydrothermal synthesis; NaBO2(H2O)2, UO3 (4:1 mol) dissolved in ultrapure H2O; pH 0.5 adjusted with concd. HNO3 (15.4 M); soln. placed in Teflon-lined Parr bomb; heated at 453 K, 48 h; cooled to ambient temp.; evapd. in a fume hood; after 2 d crystals of H3BO3-2A (triclinic) and H3BO3-3T (tetragonal) were recovered;
|
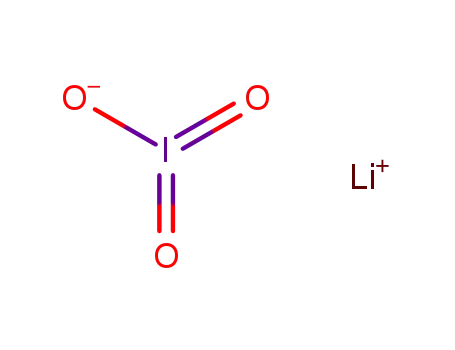
lithium iodate
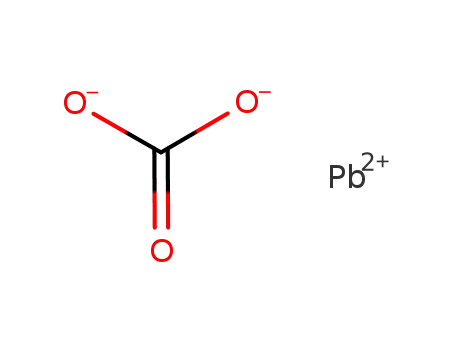
lead(II) carbonate
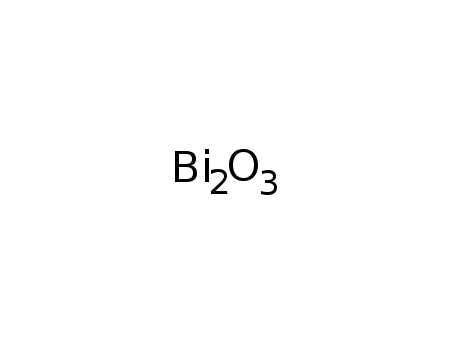
bismuth(III) oxide