Your Location:Home > Products > chemicals for Printing and dyeing > Potassium hydroxide
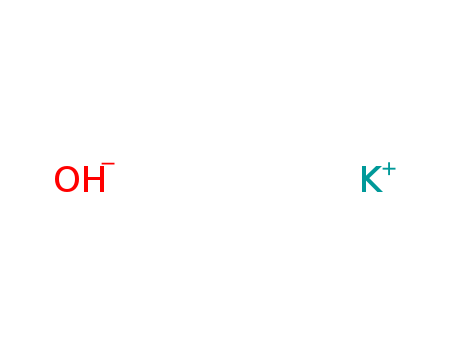


CasNo: 1310-58-3
MF: KOH
Appearance: white solid
|
Chemical Description |
Potassium hydroxide is a strong base, and trifluoroethyl ether is a solvent. |
|
Production Methods |
Potassium hydroxide is produced commerically by electrolysis of a saturated solution of potassium chloride in brine using mercury cells consisting of a titanium anode and mercury cathode. Potassium reacts with mercury forming the amalgam which, on treatment with water, forms potassium hydroxide and hydrogen. Other types of electrolytic cells, although not so commonly used today, are also known. In a diaphragm type cell that separates the cell into anode and cathode compartments, an aqueous solution of potassium chloride is electrolyzed. Potassium hydroxide and hydrogen are produced at the cathode and chlorine is liberated at the anode. The solution discharged from the cell is evaporated to concentrate potassium hydroxide and precipitate potassium chloride. Potassium hydroxide also may be made by reacting potassium superoxide with water: 2KO2 + 2H2O → 2KOH + H2O2 + O2 |
|
Reactions |
Potassium hydroxide is a very strong base, more basic than caustic soda. It is neutralized by acids. The solution on evaporation yields the corresponding potassium salt: KOH + HCl → K+ + Clˉ+ H2O Action of bromine or iodine on a warm concentrated solution of KOH forms bromate and bromide or iodate and iodide, respectively: 3Br2 + 6OHˉ→ BrO3ˉ + 5Brˉ + 3H2O 3I2 + 6OHˉ → IO3ˉ + 5Iˉ + 3H2O When carbon dioxide is passed through its aqueous solution and the solution evaporated, potassium bicarbonate is formed: KOH + CO2 → KHCO3 Reaction with carbon monoxide at 100 to 200°C at a CO pressure above 7 atm yields potassium formate: KOH + CO → HCOOK Reaction with phenol in dilute methanol solution forms potassium phenoxide: KOH + C6H5OH → C6H5OK + H2O Reaction with boric acid and hydrofluoric acid forms potassium tetrafluoroborate, KBF4: KOH + H3BO3 + 4HF → KBF4 + 4H2O An alcoholic solution of potassium hydroxide reacts with an alcoholic solution of carbon disulfide to form potassium ethylxanthogenate, C2H5OCS2K KOH + C2H5OH + CS2 → C2H5OCS2K + H2O Reaction with sodium borohydride forms potassium borohydride: POTASSIUM HYDROXIDE 759KOH + NaBH4 → KBH4 + NaOH Reaction with hydrofluoric acid forms potassium bifluoride: KOH + 2HF → KHF2 + H2O Half neutralization of a phthalic anhydride solution forms potassium hydrogen phthalate. |
|
Indications |
Potassium hydroxide (KOH) is a strong alkali that digests proteins and epidermal debris. In one study, 10% solution was applied b.i.d. to each lesion for 30 days with excellent clearance. The side effects included stinging of the lesion and one case of secondary infection. Also reported were the occurrence of a hypertrophic scar as well as some persistent or transitory hyper- and hypopigmentation. The same authors who used the 5% KOH solution completed further studies and they found it to be as effective-yet with decreased side effects. |
|
Air & Water Reactions |
Hydrolysis generates enough heat to ignite adjacent combustible material [Haz. Chem. Data 1966]. Dissolves in water (with liberation of heat, may steam and spatter. Solution is basic (alkaline). Deliquescent |
|
Reactivity Profile |
POTASSIUM HYDROXIDE absorbs moisture readily forming caustic solution that attacks aluminum and zinc. A piece of potassium hydroxide causes liquid chlorine dioxide to explode [Mellor 2:289. 1946-47]. 1,2-dichloroethylene and potassium hydroxide forms chloroacetylene, which is explosive and spontaneously flammable in air. Potassium hydroxide is highly toxic [Rutledge 1968. p. 134]. A reaction between n-nitrosomethylurea and potassium hydroxide in n-butyl ether resulted in an explosion due to the formation of diazomethane [Schwab 1972]. Potassium persulfate and a little potassium hydroxide and water ignited a polythene (polyethylene) liner of a container by release of heat and oxygen [MCA Case History 1155. 1955]. Using potassium hydroxide to dry impure tetrahydrofuran, which contains peroxides, may be hazardous. Explosions have occurred in the past. Sodium hydroxide behaves in a similar way as potassium hydroxide [NSC Newsletter Chem. Soc. 1967]. A strong base. Forms caustic solution in water. [Merck 11th ed. 1989]. |
|
Health Hazard |
Toxic by ingestion and inhalation, strong caustic, handle with gloves or tongs, corrosive to tissue. Eye, skin and upper respiratory tract irritant.Potassium hydroxide is a strongly alkaline, hydrophilic substance and therefore solid potassium hydroxide is highly corrosive. It reacts with fat and can cause irreversible damage to any site of contact with the body (for example skin or eyes). Solutions of potassium hydroxide in water at concentrations above 0.5% (w/w) are irritating at points of contact and, at higher concentrations, the solutions can be corrosive. Potassium hydroxide does not cause skin allergies. Because of the corrosive properties of potassium hydroxide, its ingestion can be fatal. Under normal conditions of handling and use, potassium hydroxide in solution will dissociate into its constituent ions and, if ingested, will not be systemically available in the body as such. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
Flammability and Explosibility |
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous solutions. |
|
Pharmaceutical Applications |
Potassium hydroxide is widely used in pharmaceutical formulations to adjust the pH of solutions. It can also be used to react with weak acids to form salts. Therapeutically, potassium hydroxide is used in various dermatological applications. |
|
Safety Profile |
Poison by ingestion. An eye irritant and severe human skin irritant. Very corrosive to the eyes, skin, and mucous membranes. Mutation data reported. Ingestion may cause violent pain in throat and epigastrium, hematemesis, collapse. Stricture of esophagus may result if substance is not immedately fatal. Above 84' it reacts with reducing sugars to form poisonous carbon monoxide gas. Violent, exothermic reaction with water. Potentially explosive reaction with bromoform + crown ethers, chlorine dioxide, nitrobenzene, nitromethane, nitrogen trichloride, peroxidized tetrahydrofuran, 2,4,6- trinitrotoluene. Reaction with ammonium hexachloroplatinate(2-) + heat forms a heat- sensitive explosive product. Violent reaction or ignition under the appropriate condtions with acids, alcohols, p-bis(l,3- dbromoethyl)benzene, cyclopentadene, germanium, hyponitrous acid, maleic anhydride, nitroalkanes, 2-nitrophenol, potassium peroxodisulfate, sugars, 2,2,3,3- tetrafluoropropanol, thorium dicarbide. When heated to decomposition it emits toxic fumes of K2O. See also SODIUM HYDROXIDE. |
|
Safety |
Potassium hydroxide is widely used in the pharmaceutical and food industries and is generally regarded as a nontoxic material at low concentrations. At high concentrations it is a corrosive irritant to the skin, eyes, and mucous membranes. (rat, oral): 0.273 g/kg |
|
Potential Exposure |
KOH is generally used as an alkali and in the manufacture of other potassium compounds. |
|
storage |
splash goggles and impermeable gloves should be worn at all times when handling these substances to prevent eye and skin contact. Operations with metal hydroxide solutions that have the potential to create aerosols should be conducted in a fume hood to prevent exposure by inhalation. NaOH and KOH generate considerable heat when dissolved in water; when mixing with water, always add caustics slowly to the water and stir continuously. Never add water in limited quantities to solid hydroxides. Potassium hydroxide should be stored in an airtight, nonmetallic container in a cool, dry place, separated from acids and incompatible substances. |
|
Shipping |
UN1814 (solution) & UN1813 (solid); Potassium hydroxide, solid or solution, Hazard class: 8; Labels: 8-Corrosive material. |
|
Purification Methods |
Its carbonate content can be reduced by rinsing KOH sticks rapidly with water prior to dissolving them in boiled out distilled water. Alternatively, a slight excess of saturated BaCl2 or Ba(OH)2 can be added to the solution which, after shaking well, is set aside so that the BaCO3 is allowed to separate out. Davies and Nancollas [Nature 165 237 1950] rendered KOH solutions carbonate free by ion exchange using a column of Amberlite IR-100 in the OH-form. |
|
Incompatibilities |
Potassium hydroxide is a strong base and is incompatible with any compound that readily undergoes hydrolysis or oxidation. Violent reaction with acids, alcohols, water, metals (when wet), halogenated hydrocarbons; maleic anhydride. Heat is generated if KOH comes in contact with water and carbon dioxide from the air. It should not be stored in glass or aluminum containers, Corrosive to zinc, aluminum, tin and lead in the presence of moisture releasing combustible/explosive hydrogen gas. Can absorb water from air and give off sufficient heat to ignite surrounding combustible materials. |
|
Waste Disposal |
Dilute with large volume of water, neutralize and flush to sewer |
|
Regulatory Status |
GRAS listed. Accepted for use in Europe in certain food applications. Included in the FDA Inactive Ingredients Database (injections, infusions, and oral capsules and solutions). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Applications |
▼▲ Industry Application Role/benefit Chemical manufacturer Manufacturer of other potassium compounds Precursor/source of potassium Electrochemistry Manufacture of batteries and fuel cells Electrolyte/ good conductors of electricity Wet processing of semiconductors Etchant/corrosivity and alkalinity Biomass fuels Manufacturing biodiesel from oils and fats Catalyst/KOH works well in the manufacture of biodiesel by transesterification of the triglycerides in vegetable oil Food Rinse or chemical peel for fruits and vegetables Additive in rinse solution/corrosivity and alkalinity Chocolate,cocoa,soft drink,ice cream,etc. Stabilizer,thickener and pH regulator Cleaning Manufacture of "potassium soaps" Saponification agent/has better softness and greater solubility than sodium soaps Industrial cleaners for oven,drain,driveway,concrete,pipe,etc. Additive/alkalinity and good solubility for grease Liquid soaps, lotions, shampoos, hairsprays, and denture cleaners Additive/helps to increase softness and solubility Medicine Disbudding calves horns and dissolving scales and hair in veterinary medicine Dissolving solution/good solubility for keratin Dissolving warts and cuticles in humans Diagnose fungal infections Diagnose agent Production of potassium boron hydrogen, spironolactone, progesterone and testosterone propionate, etc. Raw material Agriculture Potassium fertilizers (potassium phosphate) Raw material/source of potassium Paper Separation of lignin from cellulose fibers Additive/alkalinity Dyeing Manufacture of tripolycyanamide dye Raw material Textile Dyeing, bleaching and mercerizing textiles Additive/corrosivity and alkalinity Manufacture of artificial fiber and polyester fiber Main raw material Chemical analysis Titration of acids Titration agent/alkalinity Others Bleaching textiles Bleaching agent Absorption of CO2, SO3 and NO3 in gas streams Absorption agent/alkalinity Absorption of H2O Absorption agent/ hygroscopicity of anhydrous potassium hydroxide |
|
Application |
Potassium hydroxide is used as an emulsifier in lotions and as an alkali in liquid soaps, protective creams, and shaving preparations. Depending on the concentration used, it can be highly irritating to the skin and/or cause a burning sensation. It is used in making potassium salts, in electroplatingand lithography, in printing inks, as a mordantfor wood, and finds wide applicationsin organic syntheses and chemical analyses. |
|
General Description |
A white solid. Corrosive to metals and tissue. Used in soap manufacture, bleach, as an electrolyte in alkaline batteries, and as a food additive. |
InChI:InChI=1/K.H2O/h;1H2/q+1;
A process for the production of potassiu...
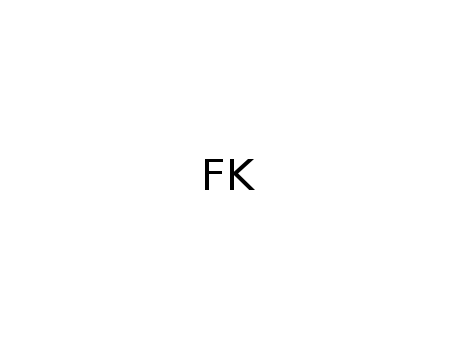
potassium fluoride

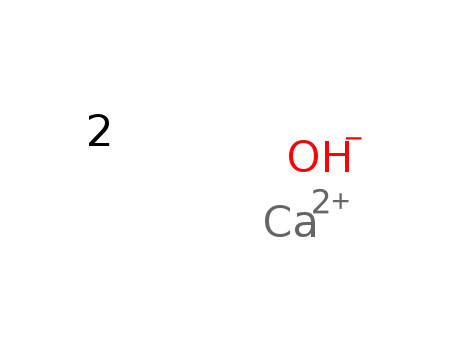
calcium hydroxide

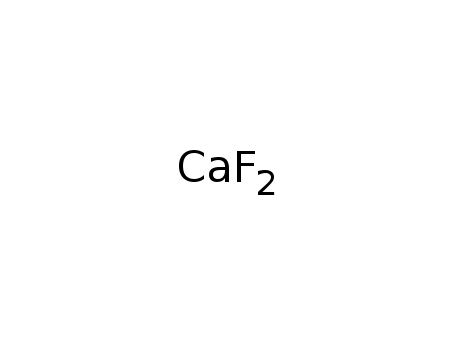
calcium fluoride

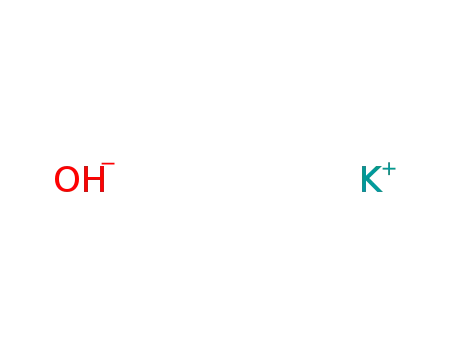
potassium hydroxide
| Conditions | Yield |
|---|---|
|
In
water;
at 70 ℃;
for 2h;
Product distribution / selectivity;
|

potassium hydroxide

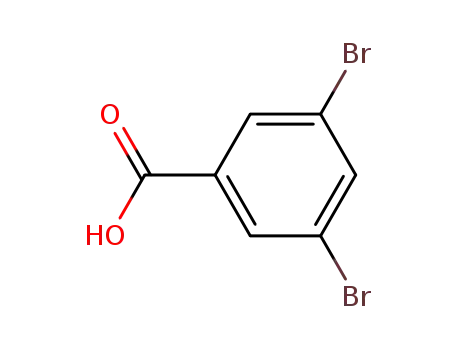
3,5-dibromobenzoic acid


argon

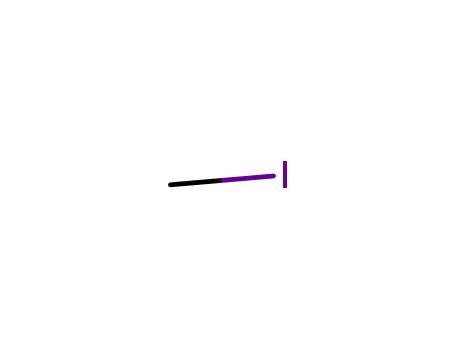
methyl iodide

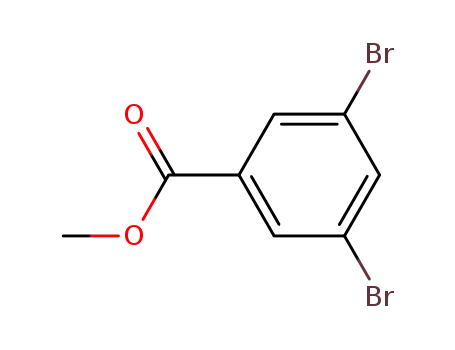
methyl 3,5-dibromobenzoate
| Conditions | Yield |
|---|---|
|
In
(2S)-N-methyl-1-phenylpropan-2-amine hydrate;
|
0.78 g (88.7%) |

potassium fluoride
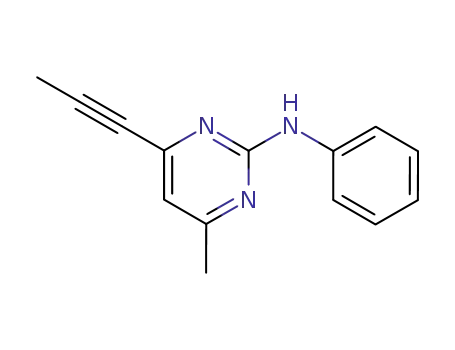
mepanipyrim
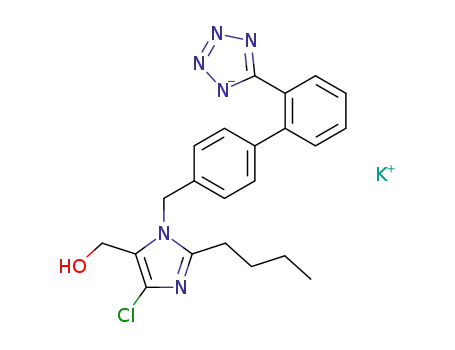
cozaar

methyl 3,5-dibromobenzoate
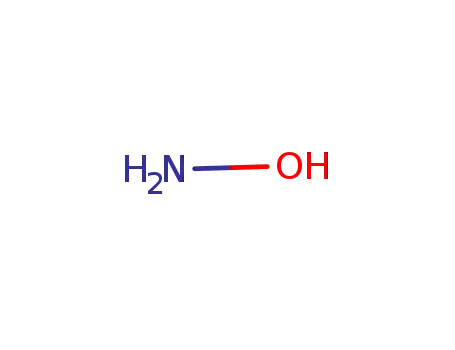
hydroxylamine